Product revenue increased 122% to $4.0 million driven by sales of KN95 masks; operating loss narrowed 21%
Conference call begins at 4:30 p.m. Eastern time today
EMERYVILLE, Calif. (August 6, 2020) – NovaBay® Pharmaceuticals, Inc. (NYSE American: NBY) reports financial results for the three and six months ended June 30, 2020 and provides a business update.
“We are reporting strong financial results for the second quarter with product revenue increasing 122% and operating loss narrowing 21% over the prior-year period, while also improving our cash position,” said Justin Hall, President and CEO of NovaBay Pharmaceuticals. “Despite difficult economic conditions in the second quarter that ravaged the economy and worsened the financial condition of many companies, we were able to substantially improve our financial results. Our strategic move last year to sell Avenova® directly to consumers online proved key to maintaining unit growth during a quarter when most of the doctors who typically prescribe Avenova were largely sidelined. Furthermore, we increased revenue by offering KN95 masks, with brisk sales during the early months of the COVID-19 pandemic.
“Given the resurgence in COVID-19 cases, consumers and physicians are responding favorably to the recently announced independent laboratory testing confirming Avenova kills SARS-CoV-2. While many disinfectants can kill the virus, only Avenova, manufactured in the USA, is completely non-toxic and gentle enough for use on the sensitive skin around the eyes, nose, and mouth, the parts of the body known to be susceptible to COVID-19 transmission,” he added. “In addition to Avenova, we are pleased to continue serving our communities during the pandemic by broadening our selection of personal protection equipment with a variety of face masks, gloves and other PPE. These products are available only in bulk quantities through Avenova.com.
“We are confident NovaBay will retain NYSE continued-listing compliance following our successful ATM financing and recent renegotiation and exercise of warrants,” he added. “With the additional capital raised, we also expect our financial resources to be sufficient to fund current operations through 2021. We have strengthened our balance sheet, brought on new product offerings and expanded our target market. The second quarter was an inflection point for our business that will serve as a springboard for accelerating future growth.”
Second Quarter Financial Results
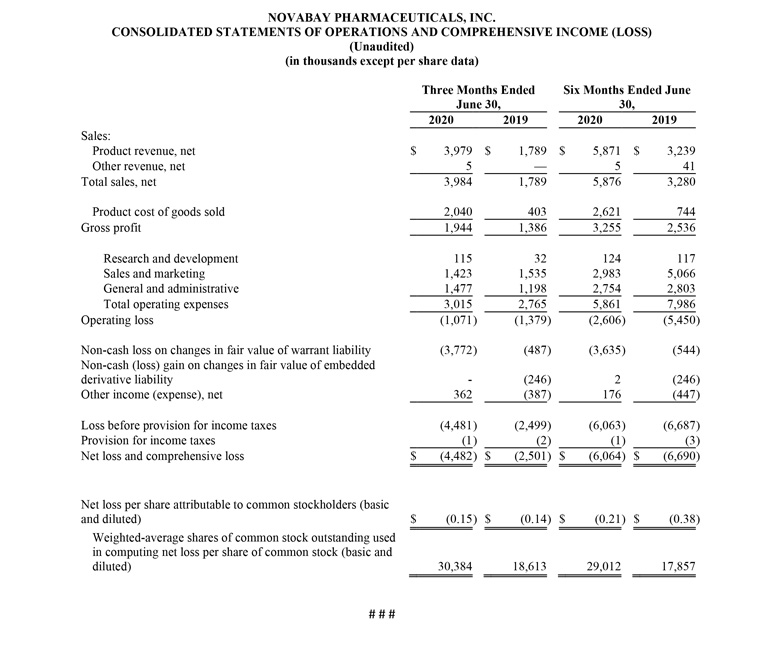
Net product revenue for the second quarter of 2020 was $4.0 million, a 122% increase from $1.8 million for the second quarter of 2019. The increase is due to $2.8 million in net product revenue from the sale of KN95 masks, with no comparable revenue in the second quarter of 2019. Avenova revenue for the second quarter of 2020 was $1.1 million, compared with $1.6 million for the second quarter of 2019. The decrease reflects an increase in Avenova units sold, offset by a lower net selling price largely due to a decrease in insurance coverage by national payors, as well as a lower net selling price associated with Avenova Direct, which was launched in June 2019.
Gross margin on net product revenue was 49% for the second quarter of 2020, compared with 77% for the prior-year period, due to the addition of the KN95 masks which are sold for a lower margin.
Operating expenses for the second quarter of 2020 were $3.0 million, compared with $2.8 million for the second quarter of 2019. Sales and marketing expenses for the second quarter of 2020 were $1.4 million, a 7% decline from $1.5 million for the second quarter of 2019, reflecting lower headcount, partially offset by an increase in digital advertising and other marketing expenses. General and administrative (G&A) expenses for the second quarter of 2020 were $1.5 million, compared with $1.2 million for the second quarter of 2019, with the increase due to higher regulatory and legal fees. Research and development (R&D) expenses for the second quarter of 2020 were $115,000, compared with $32,000 for the second quarter of 2019, mostly due to product testing and FDA applications.
Operating loss for the second quarter of 2020 was $1.1 million, a 21% improvement from the operating loss of $1.4 million for the second quarter of 2019.
Non-cash loss on the change of fair value of warrant liability for the second quarter of 2020 was $3.8 million, compared with a non-cash loss of $487,000 for the second quarter of 2019.
Loss from adjustments to the fair value of derivative liability for the second quarter of 2019 was $246,000. The Company did not record a comparable loss or gain for the second quarter of 2020.
Net other income for the second quarter of 2020 of $362,000 was primarily related to qualified expenses incurred under the Paycheck Production Program. This compares with other expense of $387,000 for the second quarter of 2019, which was due to interest expenses related to a convertible note issued March 2019.
The net loss for the second quarter of 2020 was $4.5 million, or $0.15 per share, compared with a net loss for the second quarter of 2019 of $2.5 million, or $0.14 per share.
Six Month Financial Results
Net product revenue for the six months ended June 30, 2020 was $5.9 million, a 79% increase from $3.3 million for the six months ended June 30, 2019. Gross margin on net product revenue was 55% for the first half of 2020, compared with 77% for the first half of 2019.
For the six months ended June 30, 2020, sales and marketing expenses decreased 41% to $3.0 million, while G&A expenses and R&D expenses were relatively unchanged, all compared with the six months ended June 30, 2019.
Operating loss for the first six months of 2020 was $2.6 million, a 53% improvement from the operating loss of $5.5 million for the first six months of 2019.
Non-cash loss on the change of fair value of warrant liability for the first six months of 2020 was $3.6 million, compared with a non-cash loss of $544,000 for the first six months of 2019.
Gain from adjustments to the fair value of derivative liability for the six months ended June 30, 2020 was $2,000, compared with a loss of $246,000 for the six months ended June 30, 2019.
Net other income for the first six months of 2020 was $176,000, compared with net other expense for the first six months of 2019 of $447,000.
The net loss for the six months ended June 30, 2020 was $6.1 million, or $0.21 per share, compared with a net loss for the six months ended June 30, 2019 of $6.7 million, or $0.38 per share.
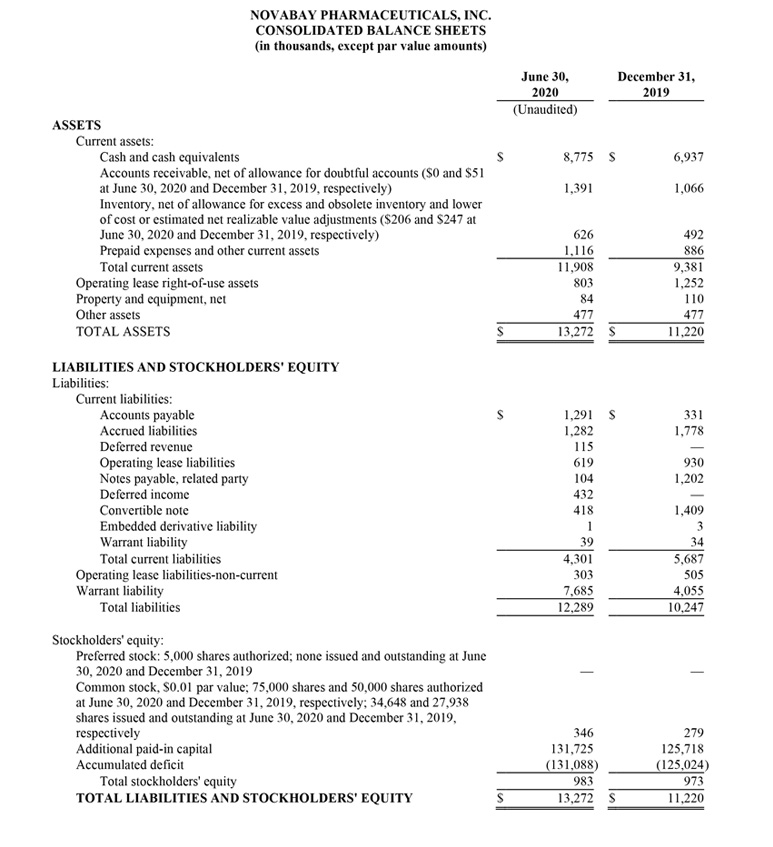
NovaBay reported cash and cash equivalents of $8.8 million as of June 30, 2020 compared with $6.9 million as of December 31, 2019. During the three months ended June 30, 2020, the Company raised net proceeds of $5.2 million from the sale of common stock through an ATM agreement. In July 2020 the Company raised net proceeds of $6.4 million through the renegotiation of warrants. This renegotiation is also expected to contribute to our initiative to successfully regain compliance with the NYSE continued-listing requirements.
Conference Call
NovaBay management will host an investment community conference call today August 6, 2020, beginning at 4:30 p.m. Eastern time (1:30 p.m. Pacific time) to discuss the Company’s financial and operational results and to answer questions. Shareholders and other interested parties may participate in the conference call by dialing 800-608-8202 from within the U.S. or 702-495-1913 from outside the U.S., with the conference identification number 7662239.
A live webcast of the call will be available at https://novabay.com/investors/events and will be archived for 90 days. A replay of the call will be available beginning two hours after the call ends through 11:59 p.m. Eastern time August 27, 2020 by dialing 855-859-2056 from within the U.S. or 404-537-3406 from outside the U.S., and entering the conference identification number 7662239.
About NovaBay Pharmaceuticals, Inc.: Going Beyond Antibiotics®
NovaBay Pharmaceuticals, Inc. is a biopharmaceutical company focusing on commercializing and developing its non-antibiotic anti-infective products to address the unmet therapeutic needs of the global, topical anti-infective market with its two distinct product categories: the NEUTROX® family of products and the AGANOCIDE® compounds. The Neutrox family of products includes AVENOVA® for the eye care market, CELLERX® for the aesthetic dermatology market and NEUTROPHASE® for the wound care market. The Aganocide compounds, still under development, have target applications in the dermatology and urology markets.
Forward-Looking Statements
Except for historical information herein, matters set forth in this press release are forward-looking within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including statements about the commercial progress and future financial performance of NovaBay Pharmaceuticals, Inc. This release contains forward-looking statements that are based upon management’s current expectations, assumptions, estimates, projections and beliefs. These statements include, but are not limited to, statements regarding our business strategies and current product offerings, potential future product offerings, possible regulatory clearance of any of our products or future products, and any future revenue that may result from selling these products, as well as generally the Company’s expected future financial results. These forward-looking statements are identified by the use of words such as “future growth,” “reduce,” and “expand,” among others. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results or achievements to be materially different and adverse from those expressed in or implied by the forward-looking statements. Factors that might cause or contribute to such differences include, but are not limited to, risks and uncertainties relating to the size of the potential market for our products, the possibility that the available market for the Company’s products will not be as large as expected, the Company’s products will not be able to penetrate one or more targeted markets, revenues will not be sufficient to meet the Company’s cash needs, and any potential regulatory problems. Other risks relating to NovaBay’s business, including risks that could cause results to differ materially from those projected in the forward-looking statements in this press release, are detailed in NovaBay’s latest Form 10-Q/K filings with the Securities and Exchange Commission, especially under the heading “Risk Factors.” The forward-looking statements in this release speak only as of this date, and NovaBay disclaims any intent or obligation to revise or update publicly any forward-looking statement except as required by law.
Socialize and Stay informed on NovaBay’s progress
Like us on Facebook
Follow us on Twitter
Connect with NovaBay on LinkedIn
Visit NovaBay’s Website
Avenova Purchasing Information
For NovaBay Avenova purchasing information:
Please call 800-890-0329 or email sales@avenova.com
www.Avenova.com
NovaBay Contact
Justin Hall
President and Chief Executive Officer
510-899-8800
jhall@novabay.com
Investor Contact
LHA Investor Relations
Jody Cain
310-691-7100
jcain@lhai.com